Abstract
Background: Four-factor prothrombin complex concentrates (4FPCCs), both non-activated and activated products, are commonly used off-label to manage oral factor Xa (FXa) inhibitor-associated severe or life-threatening bleeds including intracranial hemorrhage (ICH). Available evidence supporting 4FPCC use in the management of oral FXa inhibitor-associated ICH patients come from small-to-modest sized studies or case series. These studies or case series often differed in key inclusion and exclusion criteria employed and definition of hemostatic effectiveness utilized. We performed a systematic review and meta-analysis including multivariable meta-regression to assess the proportion of oral FXa inhibitor-associated ICH patients achieving hemostatic effectiveness with 4FPCC and the effect of key methodological decisions on this estimate.
Methods: We searched PubMed and Embase from the earliest date possible through May 31, 2022. Studies or case series were included if: 4FPCC was administered for the management of oral FXa inhibitor-associated bleeding; reported solely on ICH patients (or a subgroup of); evaluated ≥10 patients; was performed within the United States; and provided data on the proportion of patients achieving hemostatic effectiveness. Meeting abstracts were not included. We performed a random-effects meta-analysis to calculate the pooled arcsine square root transformation weighted proportion of patients achieving hemostatic effectiveness with 95% confidence intervals (CIs). Multivariable meta-regression was used to determine the impact of studies’ inclusion or exclusion of severe ICH; requirement that the oral FXa inhibitor was taken within 24-hours and/or drug concentration or FXa levels were drawn; handling of patients with missing repeat imaging; and use (or non-use) of an accepted definition of hemostatic effectiveness on our pooled estimate.
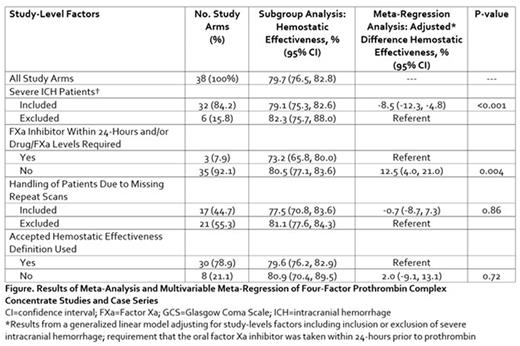
Results: We identified 36 studies or case series reporting on 38 unique study arms and including 1,732 oral FXa inhibitor-associated ICH patients managed with 4FPCC. Nearly 16% of study arms excluded patients with severe ICH (i.e., those with a hematoma volume >60 mL and/or Glasgow Coma Scale score <7) and fewer than 8% required the last oral intake of FXa inhibitor be within 24 hours or drug or FXa levels be drawn prior to 4FPCC administration. More than half (55.3%) of study arms excluded patients due to missing repeat imaging and 21.1% of arms failed to use an accepted definition to assess hemostatic effectiveness. The weighted pooled proportion of ICH patients achieving hemostatic effectiveness was 79.7% (95% CI 76.5, 82.8) (Figure). Upon multivariable meta-regression, study arms that excluded severe ICH patients reported a significant 8.5% increase in in the proportion achieving hemostatic effectiveness compared to those who retained these patients (p<0.001). Moreover, study arms that did not require oral FXa inhibitor intake within 24 hour or drug concentration/FXa levels reported a 12.5% (95% CI 4.0, 21.0, p=0.004) higher incidence of achieving hemostatic effectiveness. Exclusion of patients with missing repeat imaging and the use of an accepted definition of hemostatic effectiveness were not shown in this meta-regression analysis to significantly impact hemostatic effectiveness estimates.
Conclusions: Studies’ and case series’ exclusion of patients with severe ICH and lack of implementation of inclusion criteria to increase the likelihood of therapeutic anticoagulation being present at time of treatment may result in the overestimation of 4FPCC's hemostatic effectiveness in oral FXa inhibitor-associated ICH. Readers of these (and future) studies should consider these methodological decisions when using them in clinical decision-making.
Disclosures
Coleman:Medscape: Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding; Bayer AG: Consultancy, Honoraria, Other: Support for attending meetings and/or travel, Research Funding; Alexion Pharmaceuticals: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.